Ethics Approval of a Phase 1b Clinical Trial Investigating Deflexifol™ 1st Line Treatment of Unresectable Metastatic Colorectal Cancer
April 9, 2024
Independent endorsement for investigation of Deflexifol™ as an optimised replacement for standard of care 5-FU/LV in the 1st line treatment of…
Deflexifol™ Clinical Data Presented at the 2024 ASCO Gastrointestinal Cancers Symposium
February 2, 2024
Presenting the superior attributes of Deflexifol™ over standard of care chemotherapy formulations.
Appointment of Mr Iain Ross as Non-Executive Director
October 9, 2023
Expanding and complementing the skill set of the FivepHusion Board.
Our Focus
FivepHusion is optimising chemotherapy to improve patient treatment outcomes & quality of life.
We are developing Deflexifol™, a novel anti-cancer drug reformulation designed to address the safety and efficacy limitations of standard of care chemotherapy.
Deflexifol™ combines the chemotherapeutic drug 5-fluorouracil (5-FU) and its biomodulator leucovorin, used to improve the anti-tumour activity of 5-FU.
These drugs are commonly used to treat various solid tumours including colorectal, pancreatic, and breast cancers. The Deflexifol™ all-in-one formulation delivers both agents simultaneously at a physiological pH, thereby improving their tolerability, safety, and anti-tumour activity.
Deflexifol™
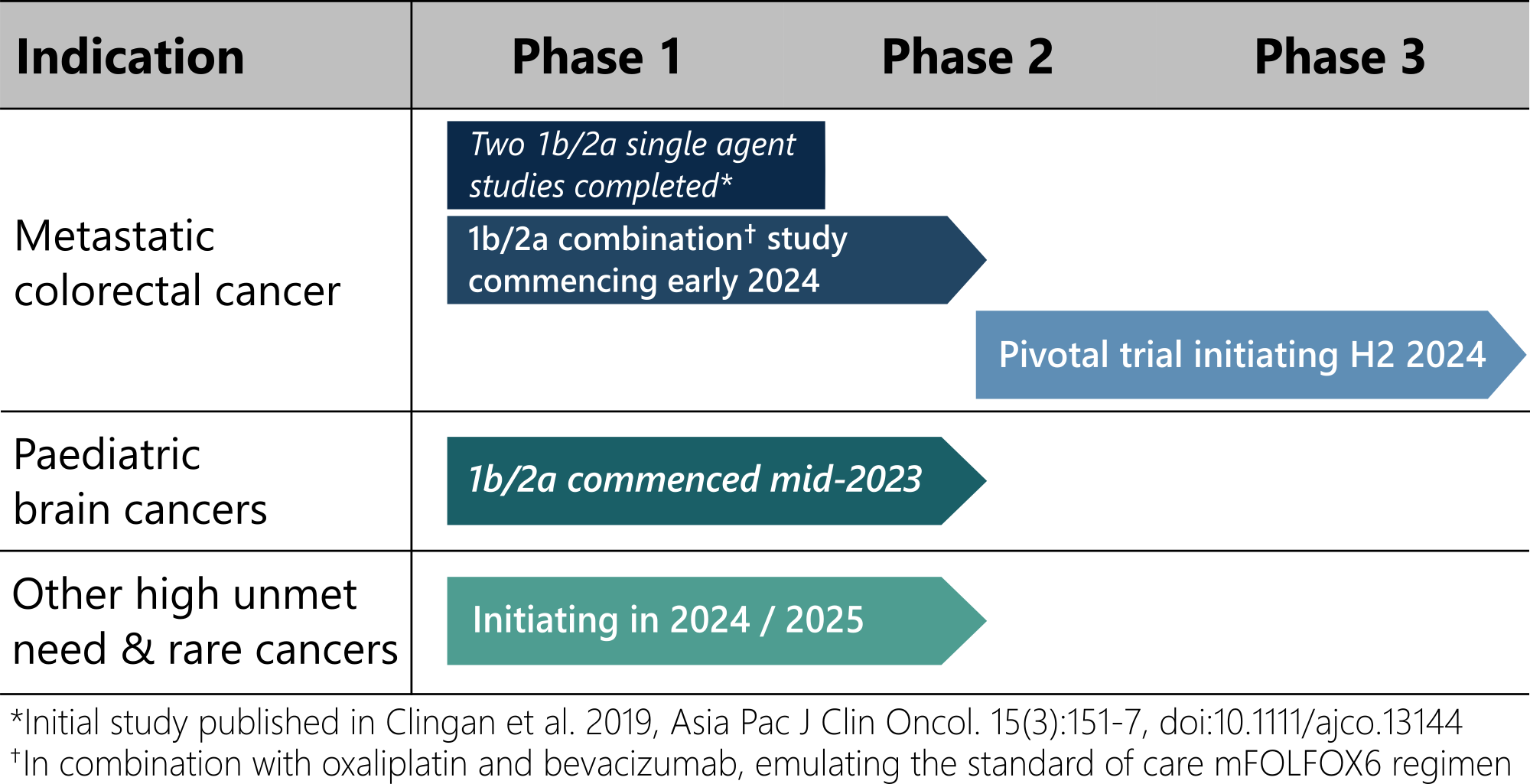
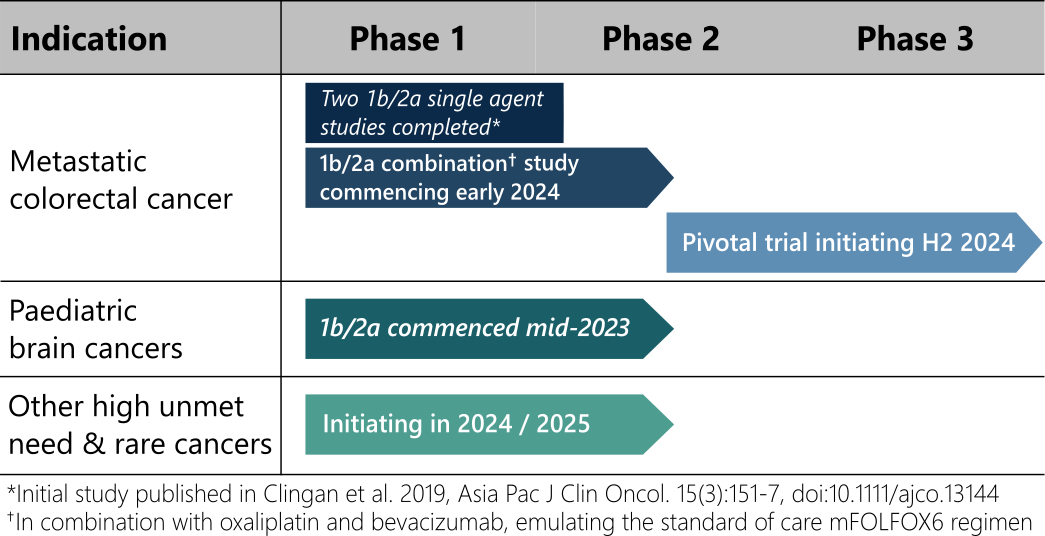
Deflexifol™ has been administered to over 58 end-stage solid tumour patients across two phase I/II clinical trials to date, and is poised to enter phase III in 2024.
Deflexifol™ is associated with significantly improved tolerability compared to standard formulations, and has demonstrated a positive safety and efficacy profile.
Deflexifol™ is positioned to replace and enhance standard of care 5-fluorouracil chemotherapy to improve patient treatment outcomes and quality of life.
As an optimised replacement of standard 5-fluorouracil chemotherapy, Deflexifol™ has the potential to improve the treatment outcomes and quality of life for patients with indications such as colorectal, pancreatic, gastric and breast cancers, which total over six million new cases globally each year.
The unique attributes of Deflexifol™ also enable potential use in patients and indications where standard 5-FU is not currently used. The use of Deflexifol™ for treating childhood brain cancers will be investigated in a phase clinical I/II trial commencing this year.
Standard 5-FU has previously demonstrated promising activity in a rare paediatric brain cancer called ependymoma, but the treatment was limited by toxicity. It is expected that the improved tolerability and efficacy of Deflexifol™ will benefit these young patients.
Deflexifol™ is being developed to address cancers with high unmet medical needs, including advanced solid tumours and paediatric brain cancers.
As an optimised replacement of standard 5-fluorouracil chemotherapy, Deflexifol™ has the potential to improve the treatment outcomes and quality of life for patients with indications such as colorectal, pancreatic, gastric and breast cancers, which total over six million new cases globally each year.
The unique attributes of Deflexifol™ also enable potential use in patients and indications where standard 5-FU is not currently used. The use of Deflexifol™ for treating childhood brain cancers is being investigated in an ongoing phase clinical I/II trial that commenced last year.
Standard 5-FU has previously demonstrated promising activity in a rare paediatric brain cancer called ependymoma, but the treatment was limited by toxicity. It is expected that the improved tolerability and efficacy of Deflexifol™ will benefit these young patients.
Deflexifol™ is being developed to address cancers with high unmet medical needs, including advanced solid tumours and paediatric brain cancers.

Our Team
Our Board
David Ranson BEng(ElecEng), Executive Chairman and Founder of FivepHusion.
An entrepreneur at heart and a director for several research and development focused companies over the past 33 years. David has worked in product development in both Australia and the USA as the director, founder and mentor of several companies in pharmaceuticals, electronics, engineering and agriculture.
Dr Christian Toouli Btech(Hons) PhD GAICD, CEO & Managing Director.
A motivated and resourceful biotechnology executive, dedicated to the development and commercialisation of breakthrough medicines for patients and to creating value for shareholders. He has 23 years’ experience in cancer research, drug development and commercialisation. Christian was the co-founder and Executive Director of Bio-Link Australia, a biotech strategic advisory and business development company, and co-founder of Tessara Therapeutics and Imunexus, biotech companies developing cutting-edge therapeutics platform technologies. Previously, Christian was a Postdoctoral Fellow at Schering-Plough Biopharma / DNAX Research Institute. He holds a PhD from the University of Sydney and was awarded a Certificate in Biotechnology Management with Honours from the University of California, Santa Cruz Extension, and First-Class Honours in Biotechnology from Flinders University of South Australia. He is also a Graduate of the Australian Institute of Company Directors.
Dr Bill Ketelbey MBBCh FFPM MBA GAICD, Executive Director.
A highly experienced and successful executive in the healthcare and pharmaceutical sector, with more than 30 years’ experience leading the development of numerous drugs across a broad range of therapeutic areas. Bill is a Non-Executive Director and Head of the Commercialisation Committee of the Westmead Institute of Medical Research. Previously, he was CEO & Managing Director of Actinogen Medical Ltd (ASX:ACW), and Pfizer Regional Vice President APAC, Senior Vice-President/ Senior Medical Director Japan and Country Medical Director ANZ.
Bill has a medical degree from the University of the Witwatersrand (South Africa), is a Fellow of the Faculty of Pharmaceutical Medicine with the Royal College of Physicians (UK), has an MBA from Macquarie University (Australia), and is a Graduate of the Australia Institute of Company Directors.
Iain Ross BSc Hons, CDir (IoD), Non-Executive Director.
Mr. Ross has over 40 years’ experience in the international life sciences and technology sectors and has held significant roles in multi-national companies including Sandoz, Hoffman La Roche, Reed Business Publishing and Celltech Group plc. He has completed multiple financing transactions and has over 30 years’ experience in cross-border management as a chairman and CEO. He has led and participated in eight Initial Public Offerings (IPOs) and has direct experience of M&A transactions in Europe, the USA and the Pacific Rim.
Mr. Ross currently serves as Chairman of Silence Therapeutics plc (Nasdaq), Executive Chairman of ReNeuron Group plc (LSE) and he is a Non-Executive Director of BiVictrix Therapeutics plc (LSE). He also advises a number of private companies in the biotechnology sector. He is a qualified Chartered Director and former Vice Chairman of the Council of Royal Holloway, London University.
Clinical Advisory Board
Professor Stephen Clarke OAM, MBBS PhD MD FRACP FAChPM FAHMS, CAB Chairman. A medical oncologist at Royal North Shore Hospital in Sydney; Professor of Medicine at the University of Sydney; and Chief Medical Officer, Medical Oncology, at Genesis Care.
Professor John Simes AO, MBBS SM BSc(Med) MD FRACP FAHMS. A Senior Principal Research Fellow, National Health and Medical Research Council (NHMRC) and Senior Associate Director of the NHMRC Clinical Trials Centre, University of Sydney. He is a practicing medical oncologist at the Chris O’Brien Lifehouse and Royal Prince Alfred Hospital specialising in neuro-oncology.
Professor Andrew McLachlan AM, BPharm (Hons) PhD FPS FACP MSHPA MCPA. A pharmacist, academic and researcher with experience in clinical pharmacology and the quality use of medicines. He is Head of School and Dean at the Sydney Pharmacy School, The University of Sydney, and consultant to Bellbery Ltd and various TGA committees.
Professor John Zalcberg AO, BBS PhD FRACP FRACMA FAHMS FAICD. Head of the Cancer Research Program in the School of Public Health and Preventive Medicine at Monash University. He holds the inaugural Tony Charlton Chair in Cancer Research at the Alfred Hospital, Melbourne, Australia.
Paediatric Advisor
Associate Professor David Ziegler MD/PhD, FRACP, Diploma Paediatrics MBBS, BSc (Medicine) FivepHusion Paediatric Oncology Advisor and Senior Staff Specialist in the Kids Cancer Centre at Sydney Children’s Hospital. David has expertise in neuro-oncology and early phase clinical trials. He completed his clinical training in paediatric haematology and oncology at Sydney Children’s Hospital. From 2005-2007 he was a Fulbright Scholar at the Dana Farber Cancer Institute, Harvard Medical School and Children’s Hospital Boston. He is a Conjoint Associate Professor at the University of New South Wales and a Group Leader at the Children’s Cancer Institute (CCI), where his preclinical research focuses on novel therapies for childhood brain tumours. He is deputy director of the Kids Cancer Alliance – a translational research program supported by the Cancer Institute NSW.
Founder Advisors
Professor Philip Clingan OAM, D.Sc. (H.C.); MBBS. (Hons.); F.R.A.C.P. Founder of FivepHusion, a Medical Oncologist with over 30 years experience in clinical practice, and a research fellow at Illawarra Health and Medical Research Institute, University of Wollongong.
He currently consults at Southern Cancer Institute and SMDCC Pty Ltd (A Private Day Only Hospital). He is interested in all cancer types, and specialises in the treatment of breast, lung and colorectal cancers. He is actively involved in clinical research having contributed to over 120 international clinical trials.
Professor Marie Ranson, BSc (Hons, Biochemistry) UNSW; PhD, University of Sydney. Founder of FivepHusion and Senior Professor of University of Wollongong, School of Chemistry and Molecular Bioscience, Molecular Horizons, Faculty of Science, Medicine and Health. Marie is also the Illawarra Lead and Deputy Director of the Cancer Institute NSW funded CONCERT Translational Cancer Research Centre. She has held appointments as the Cancer Scientific Director at the Illawarra Health and Medical Research Institute, acting Head of School of Biological Sciences and held a Cancer Institute Career Development Fellowship. Prior to this, she held a Fogarty post-doctoral fellowship at the National Institutes of Health, Bethesda MD, USA. Her research interests are the molecular biomarkers of cancer invasion and metastasis and she has published greater than 110 publications.
Emeritus Professor John Bremner AM was Professor of Organic Chemistry in the Department of Chemistry, University of Wollongong from 1991-2007, and is currently an Emeritus Professor of the University. A founder of FivepHusion, his research interests cover medicinal chemistry (particularly new anti-bacterial and anti-cancer agents), heterocyclic chemistry, and natural products. In 2001 he received the Adrien Albert Award of the RACI’s Division of Biomolecular Chemistry for his contributions to medicinal chemistry, and received the Halpern Medal (School of Chemistry, Wollongong) in 2008. He is a Fellow of The Royal Australian Chemical Institute (Distinguished Fellowship Award 2011) and a Fellow of The Royal Society of Chemistry.
Pipeline
More about us
FivepHusion is a private Australian biotechnology company headquartered in New South Wales, founded in 2018.
FivepHusion is developing Deflexifol™, a novel drug formulation created to address oncologists’ needs for safer and more efficacious chemotherapy. Deflexifol™ research and development was originally conducted at the University of Wollongong (UoW), with IP assigned to FivepHusion. The company maintains an ongoing collaboration with UoW.
FivepHusion’s vision is to optimise standard of care cancer therapy to improve patient outcomes and quality of life, leading to a better future for patients.
Contact & Connect
Follow us on LinkedIn for news and updates

Partners & affiliates























